What does ‘speed to cycle’ mean for the pharmaceutical industry and why does it matter so much?

What is ‘speed to cycle’?
Speed to cycle is the amount of time it takes going from decision point to decision point in the drug development pipeline. It is typically referred to in the context of trying to compress this time and thus reduce speed to cycle. You can see many examples of the ‘decision points’ across the drug development pipeline in the image below; including which targets to validate in the wet lab, how and what evidence is put together for FDA submissions, etc.
Pharmas already have standardised protocols to help them get to the right decision, but it takes them a long time to get there. Speed to cycle is all about maintaining the quality of the decision, and reducing time to get there.
Enhancing speed to cycle is critical to give pharma companies longer commercial use of their patents. While reducing failure rate in pharma is all about making the right decision, improving speed to cycle is about making the right decision faster. Reducing failure rate combined with improving speed to cycle is a critical way forward for a pharma organisation to come out on top in an increasingly competitive landscape.
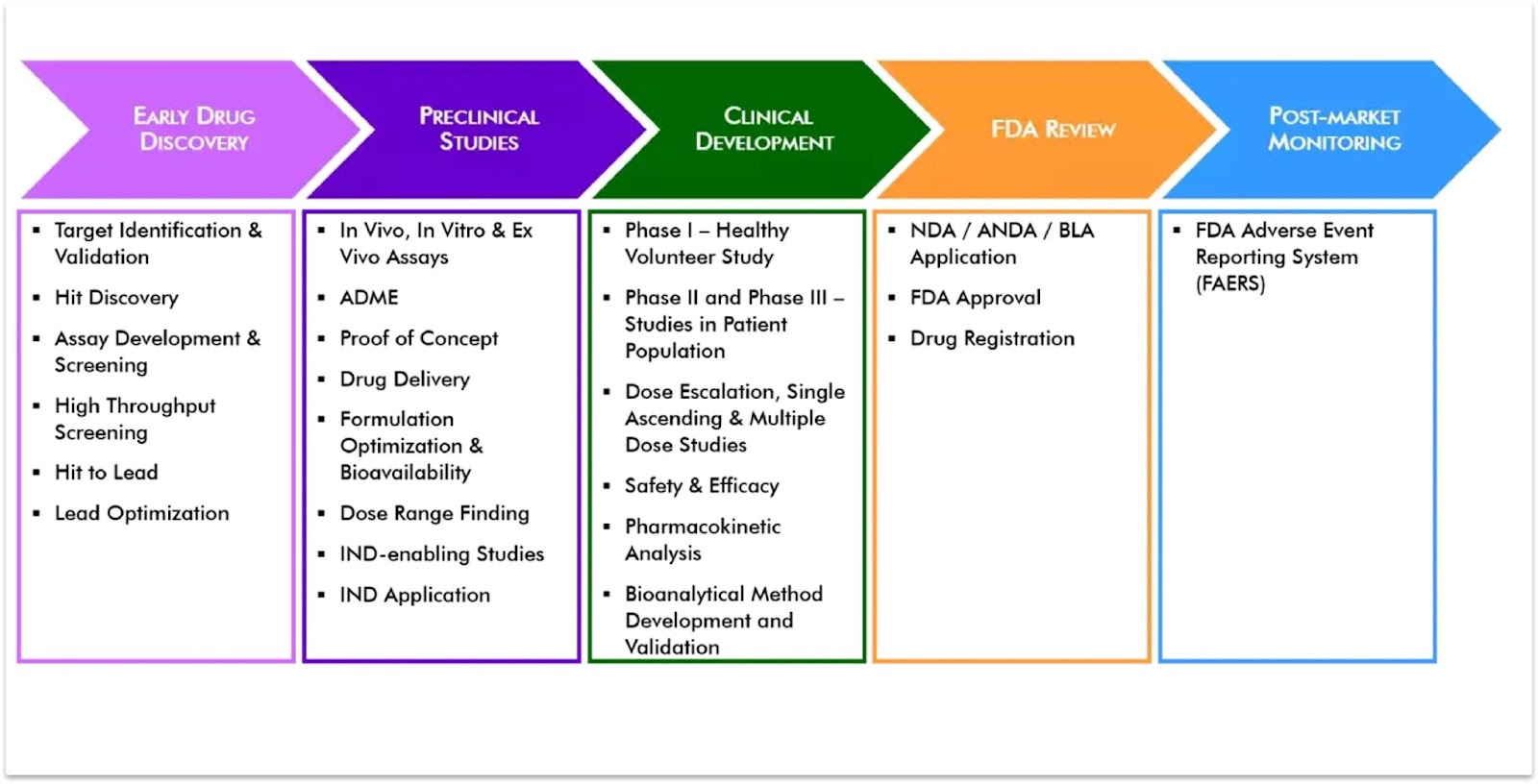
Image source: NorthEast BioLab Phases of Drug Development Process, Drug Discovery Process | NorthEast BioLab (nebiolab.com) Image link: https://www.nebiolab.com/wp-content/uploads/2019/09/Drug-Development-Summary.webp
Speeding up one component doesn’t do much in itself as so many things happen in parallel in drug development; so pharma at organisational and departmental levels need to speed up several aspects at once to see any true gains in quickly making decisions.
Finding targets is great, but failing things fast and early in the pipeline is even better and initiatives to achieve both of these things in parallel is a good example of doing multiple things to reduce speed to cycle in parallel.
It’s all about truth-seeking behaviours
Truth-seeking behaviours are concerned with collecting all the evidence that is available around something to, for example, understand the truth about a particular target to make the right decision. The AstraZeneca 5R framework is a great example of what truth-seeking behaviour standards should look like. If truth-seeking behaviours are applied across the whole pipeline to make an informed decision at every stage quickly, this would be the key to true speed to cycle enhancement.
Why is improving speed to cycle a critical strategic objective for large pharma?
The average expenditure to introduce a novel drug to market is staggering, ranging from about $2.5 to about $6.16 billion total R&D expenditures per new drug. (Source: Analysis of pharma R&D productivity - a new perspective needed https://pubmed.ncbi.nlm.nih.gov/37506762/).
This figure is experiencing a consistent upward trend (See High drug prices are not justified by industry’s spending on research and development https://www.bmj.com/content/380/bmj-2022-071710).
Speed to cycle is important because a few days or a week saved for each decision gate mounts up to weeks or months saved across the drug development pipeline. This in turn extends the period when a pharma can recover these increasing R&D costs and make a profit before competition kicks in.
What efficiencies and cost savings can be introduced to improve speed to cycle?
The list is infinite and spans things every large pharmaceutical organisation in the industry could and should be improving, as well as organisation-specific efficiencies.
So what are the obvious wins we can make with the modern tools and capabilities available to us?
One of the reasons Artificial Intelligence (AI) is such a hot topic in biopharma now is that it has the potential to serve as a critical tool in addressing the myriad of challenges that occur throughout the drug discovery pipeline and thus improve speed to cycle. However, it's crucial to understand that AI isn't a universal solution – it’s effectiveness can vary, depending on the particular challenge in question.
We at Biorelate are helping pharma companies make better decisions faster by extracting cause-and-effect relationships hidden in the biomedical literature.
For our data scientist customers, this means additional high quality edges for their knowledge graphs, reducing the time spent on text curation, and increasing focus on more value-add activities such as data analysis.
For our bioinformatician customers, it means they can more quickly generate hypotheses for target ID, for example.
For our customers spanning biologists and therapeutic subject matter experts, it means less time trawling the papers to understand the mechanism that underpins an experimental observation, and less time pinpointing the relevant next steps.
Take these cause-and-effect data capabilities further down the pipeline, and you get potential acceleration and speed to cycle improvements for decisions around safety assessment, safety prediction, patient selection, target druggability, hit-to-lead, lead optimisation, planning first-in-human studies, market assessment, methods development, etc.
We work with a large pharma where in minutes rather than weeks we were able to propose plausible mechanisms that explained the effect seen in preclinical safety studies; provide the supporting evidence; and suggest pharmacodynamic biomarkers that could be used to validate the mechanism in the lab.
Advanced cause-and-effect data curation has the potential, when applied to use cases throughout an entire drug development pipeline, to improve speed to cycle to save a single pharmaceutical organisation months across discovery.
If you’d like to discuss further the use cases for cause-and-effect advanced data curation or request a demo of Galactic AI™, the most novel knowledge graph in biopharma, contact us directly at info@biorelate.com
Latest News
Discover new insights and updates for data science in biopharma






